INFUSION EXPERIENCE
INFUSION EXPERIENCE
INFUSION EXPERIENCE

BRIUMVI is given as a 1-hour infusion, twice per year*
*Following the starting dose.
BRIUMVI is an infusion therapy that is administered by a healthcare provider (such as a doctor or nurse) through a needle placed in a vein in your arm, also known as an intravenous (IV) infusion. Your healthcare provider is there to support you by reviewing the overall treatment process, answering any questions you may have, and monitoring for potential infusion-related reactions. Please allow enough time in your schedule for your infusion treatments.
What to expect on a typical BRIUMVI infusion day
Pre-infusion
30-60 MINUTES
Prior to every infusion, your healthcare provider will evaluate your overall health and answer any questions you may have about your infusion treatment.
Pre-infusion medication may be given orally or intravenously (IV) to help reduce possible infusion-related reactions.
During infusion
1 HOUR
Infusions (following the starting dose) will typically take 1 hour.
Post-infusion
FLEXIBLE
For your first 2 infusions, your healthcare provider will observe you for 1 hour after your infusion is completed.
Post-infusion monitoring is at your healthcare provider’s discretion and is not required after your third infusion and beyond (unless you have experienced an infusion-related reaction and/or signs of hypersensitivity with your current or prior infusions).
95% of all BRIUMVI 1-hour infusions were completed in 1 hour† without interruption in clinical trials
†+/- 5 minutes
BRIUMVI offers a dosing schedule that may help provide more time for you and less time for your infusion
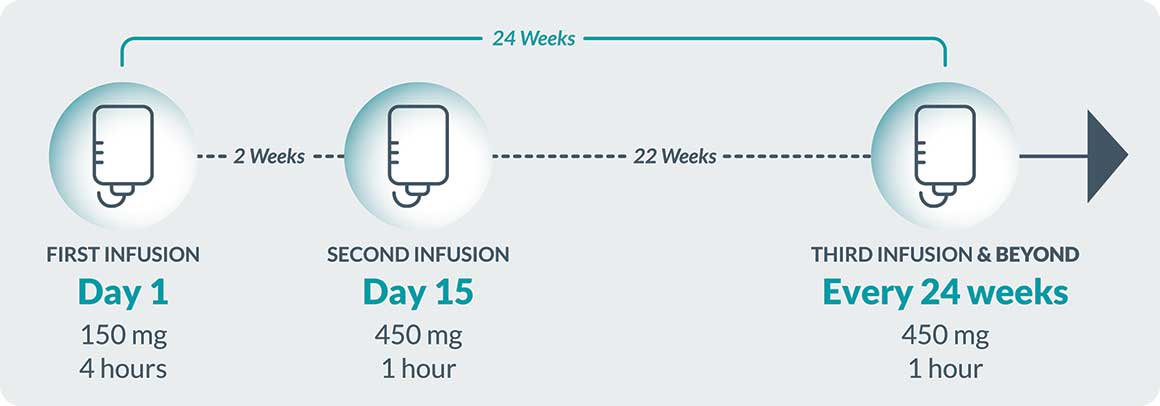
First Infusion
Also known as your starting dose, your first infusion will last about 4 hours excluding any pre-infusion medication or post-infusion monitoring.
Second Infusion
It will be given 2 weeks after your Day 1 starting dose and will last about 1 hour, excluding any pre-infusion medication or post-infusion monitoring.
Third Infusion & Beyond
Will be given every 24 weeks (about 6 months) following your Day 1 starting dose. These infusions will also last about 1 hour. Post-infusion monitoring is at your healthcare provider’s discretion and is not required after your third infusion and beyond (unless you have experienced an infusion-related reaction and/or signs of hypersensitivity with your current or prior infusions).
See what BRIUMVI Patient Support can
offer you
Understanding the safety and side effects of BRIUMVI
JOIN THE THOUSANDS WHO ARE TAKING ON RMS WITH BRIUMVI
Discover how BRIUMVI is impacting real people with RMS.
Hear from people who have included it in their RMS treatment journey.

JOIN THE THOUSANDS WHO ARE TAKING ON RMS WITH BRIUMVI

Discover how BRIUMVI is impacting real people with RMS.
Hear from people who have included it in their RMS treatment journey.
JOIN THE THOUSANDS WHO ARE TAKING ON RMS WITH BRIUMVI
Discover how BRIUMVI is impacting real people with RMS.
Hear from people who have included it in their RMS treatment journey.

People featured on this page are taking BRIUMVI and have been compensated by TG Therapeutics for their time.
INDICATION AND IMPORTANT
SAFETY INFORMATION
Who should not receive BRIUMVI?
What is the most important information I should know about BRIUMVI?
- fever
- chills
- headache
- flu-like symptoms
- fast heartbeat
- hives
- itchy skin
- dizziness
- feeling faint
- swelling of tongue or throat
- trouble breathing
- wheezing
- nausea
- abdominal pain
- throat irritation
- redness of the face or skin
These infusion reactions can happen over 24 hours after your infusion. It is important that you call your healthcare provider right away if you get any of the signs or symptoms listed above after each infusion. If you get an infusion reaction, your healthcare provider may need to stop or slow down the rate of your infusion.
- Infection:
- Infections are a common side effect, and upper respiratory tract infections are one of the most common side effects of BRIUMVI. BRIUMVI increases your risk of getting infections caused by bacteria or viruses that may be life-threatening or cause death. Tell your healthcare provider if you have an infection or have any of the following signs of infection including fever, chills, a cough that does not go away, or painful urination. Your healthcare provider should delay your treatment with BRIUMVI until your infection is gone.
- Hepatitis B virus (HBV) reactivation: Before starting treatment with BRIUMVI, your healthcare provider will do blood tests to check for hepatitis B viral infection. If you have ever had hepatitis B virus infection, the hepatitis B virus may become active again during or after treatment with BRIUMVI. Hepatitis B virus becoming active again (called reactivation) may cause serious liver problems including liver failure or death. Your healthcare provider will monitor you if you are at risk for hepatitis B virus reactivation during treatment and after you stop receiving BRIUMVI.
- Weakened immune system: BRIUMVI taken before or after other medicines that weaken the immune system could increase your risk of getting infections.
- Progressive Multifocal Leukoencephalopathy (PML): PML may happen with BRIUMVI. PML is a rare, serious brain infection caused by a virus that may get worse over days or weeks. PML can result in death or severe disability. Tell your healthcare provider right away if you have any new or worsening neurologic signs or symptoms. These symptoms may include weakness on one side of your body, loss of coordination in arms and legs, vision problems, changes in thinking and memory which may lead to confusion, and personality changes.
- Low immunoglobulins: BRIUMVI may cause a decrease in some types of antibodies. Your healthcare provider will do blood tests to check your blood immunoglobulin levels.
Before receiving BRIUMVI, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of BRIUMVI?
Indication



