WHAT IS BRIUMVI?
BRIUMVI is the only B‑cell therapy approved for people with RMS that is administered in a 1‑HOUR INFUSION, TWICE PER YEAR.* If you are starting or switching treatment, there are many factors to consider. Learn more about BRIUMVI as a treatment option and talk to your healthcare provider about whether it might be right for you.
*Following the starting dose.
WHAT IS BRIUMVI?
BRIUMVI is the only B‑cell therapy approved for people with RMS that is administered in a 1‑HOUR INFUSION, TWICE PER YEAR.* If you are starting or switching treatment, there are many factors to consider. Learn more about BRIUMVI as a treatment option and talk to your healthcare provider about whether it might be right for you.
*Following the starting dose.
WHAT IS BRIUMVI?
BRIUMVI is the only B‑cell therapy approved for people with RMS that is administered in a 1‑HOUR INFUSION, TWICE PER YEAR.* If you are starting or switching treatment, there are many factors to consider. Learn more about BRIUMVI as a treatment option and talk to your healthcare provider about whether it might be right for you.
*Following the starting dose.

Why Choose BRIUMVI

Proven results in 2 separate,
2-year clinical studies
Reduced relapses by 59% in Study 1 (in Study 2 by 49%) compared to teriflunomide†
Significantly reduced brain lesions on magnetic resonance imaging (MRI)

BRIUMVI Safety Profile
Tested in 2 clinical studies with more than 1,000 people, of whom 545 were given BRIUMVI
Overall infection rates of BRIUMVI and teriflunomide were similar. Most were mild to moderate and consisted of upper respiratory tract infections and urinary tract infections

1-hour, twice-per-year* infusion option
The only available 1-hour, twice-per-year* IV infusion following the starting dose
Post-infusion monitoring is at your healthcare provider’s discretion and is not required after your third infusion and beyond (unless you have experienced an infusion-related reaction and/or signs of hypersensitivity with your current or prior infusions)
*Following the starting dose.
†Teriflunomide is the active ingredient in AUBAGIO®. AUBAGIO® is a registered trademark of Sanofi or an affiliate.
IV, intravenous

Proven results in 2 separate,
2-year clinical studies
Reduced relapses by 59% in Study 1 (in Study 2 by 49%) compared to teriflunomide†
Significantly reduced brain lesions on magnetic resonance imaging (MRI)

BRIUMVI Safety Profile
Tested in 2 clinical studies with more than 1,000 people, of whom 545 were given BRIUMVI
Overall infection rates of BRIUMVI and teriflunomide were similar. Most were mild to moderate and consisted of upper respiratory tract infections and urinary tract infections

1-hour, twice-per-year* infusion option
The only available 1-hour, twice-per-year* IV infusion following the starting dose
Post-infusion monitoring is at your healthcare provider’s discretion and is not required after your third infusion and beyond (unless you have experienced an infusion-related reaction and/or signs of hypersensitivity with your current or prior infusions)
*Following the starting dose.
†Teriflunomide is the active ingredient in AUBAGIO®. AUBAGIO® is a registered trademark of Sanofi or an affiliate.
IV, intravenous
Looking for more information on BRIUMVI?
Download the BRIUMVI Patient Brochure
Looking for more information on BRIUMVI?
Download the BRIUMVI Patient Brochure
Looking for more information on BRIUMVI?
Download the BRIUMVI Patient Brochure
How BRIUMVI works
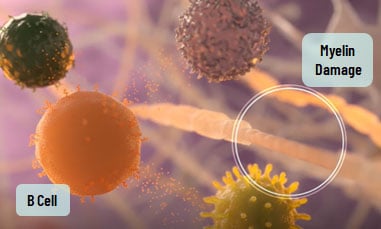
What are B Cells?
B cells are a type of white blood cell that play a role in the immune system. In people with RMS, B cells are thought to contribute to the immune system’s attack on myelin. Myelin is the protective covering around nerves. Damage to myelin can disrupt communication between the brain and the rest of the body.
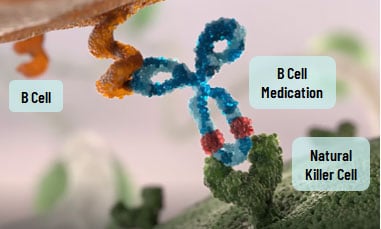
How do b-cell treatments work?
B-cell treatments work by binding to B cells and interacting with natural killer (NK) cells, another type of white blood cell that helps remove B cells from circulation.
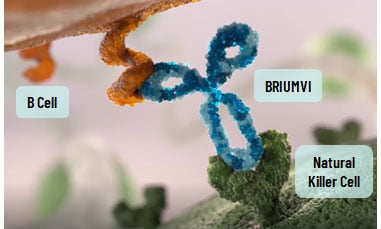
How does BRIUMVI work?
BRIUMVI is designed to specifically target B cells and facilitate their removal. Although it is not known exactly how BRIUMVI works, it is thought to achieve this by creating a strong connection with NK cells, and as a result it enhances the process by which B cells are removed.
Medications that target B cells have been available to treat diseases for more than 25 years and are the most widely prescribed type of medication to treat RMS today.
BRIUMVI was approved by the FDA in 2022
Learn more about the results from the head‑to‑head clinical studies
What to expect on your BRIUMVI
infusion day
JOIN THE THOUSANDS WHO ARE TAKING ON RMS WITH BRIUMVI
Discover how BRIUMVI is impacting real people with RMS.
Hear from people who have included it in their RMS treatment journey.

JOIN THE THOUSANDS WHO ARE TAKING ON RMS WITH BRIUMVI

Discover how BRIUMVI is impacting real people with RMS.
Hear from people who have included it in their RMS treatment journey.
JOIN THE THOUSANDS WHO ARE TAKING ON RMS WITH BRIUMVI
Discover how BRIUMVI is impacting real people with RMS.
Hear from people who have included it in their RMS treatment journey.

People featured on this page are taking BRIUMVI and have been compensated by TG Therapeutics for their time.
INDICATION AND IMPORTANT
SAFETY INFORMATION
Who should not receive BRIUMVI?
What is the most important information I should know about BRIUMVI?
- fever
- chills
- headache
- flu-like symptoms
- fast heartbeat
- hives
- itchy skin
- dizziness
- feeling faint
- swelling of tongue or throat
- trouble breathing
- wheezing
- nausea
- abdominal pain
- throat irritation
- redness of the face or skin
These infusion reactions can happen over 24 hours after your infusion. It is important that you call your healthcare provider right away if you get any of the signs or symptoms listed above after each infusion. If you get an infusion reaction, your healthcare provider may need to stop or slow down the rate of your infusion.
- Infection:
- Infections are a common side effect, and upper respiratory tract infections are one of the most common side effects of BRIUMVI. BRIUMVI increases your risk of getting infections caused by bacteria or viruses that may be life-threatening or cause death. Tell your healthcare provider if you have an infection or have any of the following signs of infection including fever, chills, a cough that does not go away, or painful urination. Your healthcare provider should delay your treatment with BRIUMVI until your infection is gone.
- Hepatitis B virus (HBV) reactivation: Before starting treatment with BRIUMVI, your healthcare provider will do blood tests to check for hepatitis B viral infection. If you have ever had hepatitis B virus infection, the hepatitis B virus may become active again during or after treatment with BRIUMVI. Hepatitis B virus becoming active again (called reactivation) may cause serious liver problems including liver failure or death. Your healthcare provider will monitor you if you are at risk for hepatitis B virus reactivation during treatment and after you stop receiving BRIUMVI.
- Weakened immune system: BRIUMVI taken before or after other medicines that weaken the immune system could increase your risk of getting infections.
- Progressive Multifocal Leukoencephalopathy (PML): PML may happen with BRIUMVI. PML is a rare, serious brain infection caused by a virus that may get worse over days or weeks. PML can result in death or severe disability. Tell your healthcare provider right away if you have any new or worsening neurologic signs or symptoms. These symptoms may include weakness on one side of your body, loss of coordination in arms and legs, vision problems, changes in thinking and memory which may lead to confusion, and personality changes.
- Low immunoglobulins: BRIUMVI may cause a decrease in some types of antibodies. Your healthcare provider will do blood tests to check your blood immunoglobulin levels.
Before receiving BRIUMVI, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of BRIUMVI?
Indication



